Important Safety Information
WARNING: CENTRAL NERVOUS SYSTEM (CNS) DEPRESSION and ABUSE AND MISUSE
Central Nervous System Depression
LUMRYZ™ (sodium oxybate) is a CNS depressant. Clinically significant respiratory depression and obtundation may occur in patients treated with LUMRYZ at recommended doses. Many patients who received LUMRYZ during clinical trials in narcolepsy were receiving CNS stimulants.
Abuse and Misuse
LUMRYZ (sodium oxybate) is the sodium salt of gamma-hydroxybutyrate (GHB). Abuse or misuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreased consciousness, coma, and death.
Because of the risks of CNS depression and abuse and misuse, LUMRYZ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the LUMRYZ REMS.
INDICATIONS AND USAGE
LUMRYZ (sodium oxybate) for extended-release oral suspension is a central nervous system depressant indicated for the treatment of cataplexy or excessive daytime sleepiness (EDS) in adults with narcolepsy.
CONTRAINDICATIONS
LUMRYZ is contraindicated for use in:
- combination with sedative hypnotics or alcohol
- patients with succinic semialdehyde dehydrogenase deficiency
WARNINGS AND PRECAUTIONS
Central Nervous System Depression
The concurrent use of LUMRYZ with other CNS depressants, including but not limited to opioid analgesics, benzodiazepines, sedating antidepressants or antipsychotics, sedating antiepileptic drugs, general anesthetics, muscle relaxants, and/or illicit CNS depressants, may increase the risk of respiratory depression, hypotension, profound sedation, syncope, and death. If use of these CNS depressants in combination with LUMRYZ is required, dose reduction or discontinuation of one or more CNS depressants (including LUMRYZ) should be considered. In addition, if short-term use of an opioid (eg, post- or perioperative) is required, interruption of treatment with LUMRYZ should be considered.
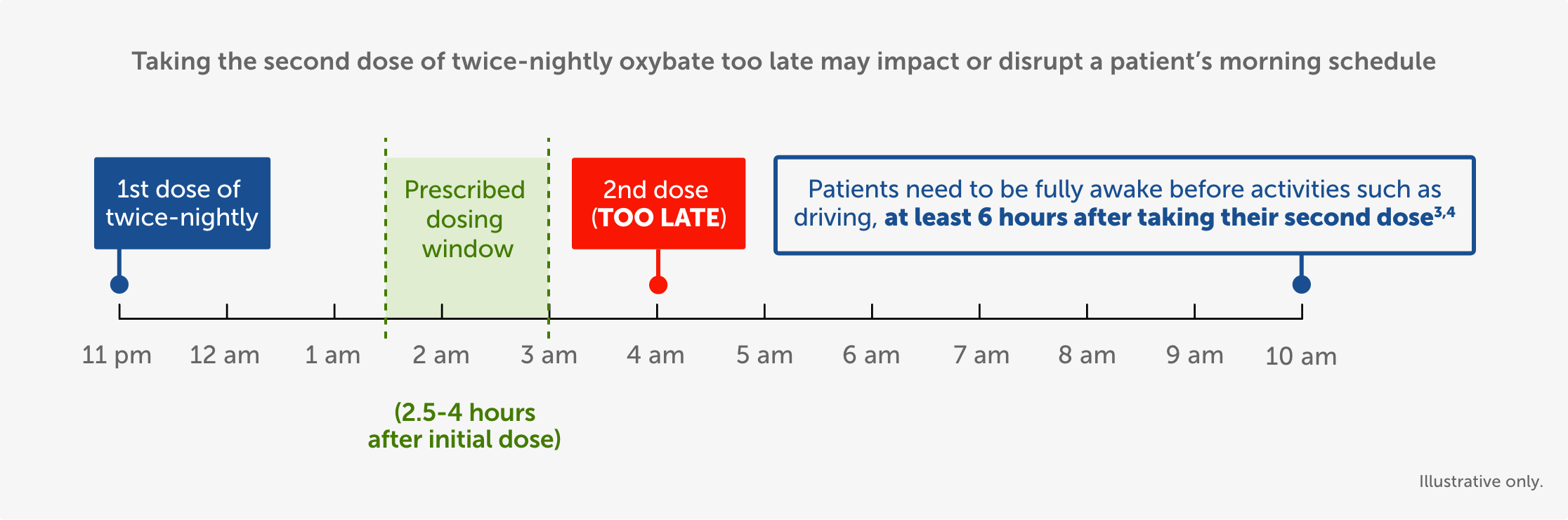
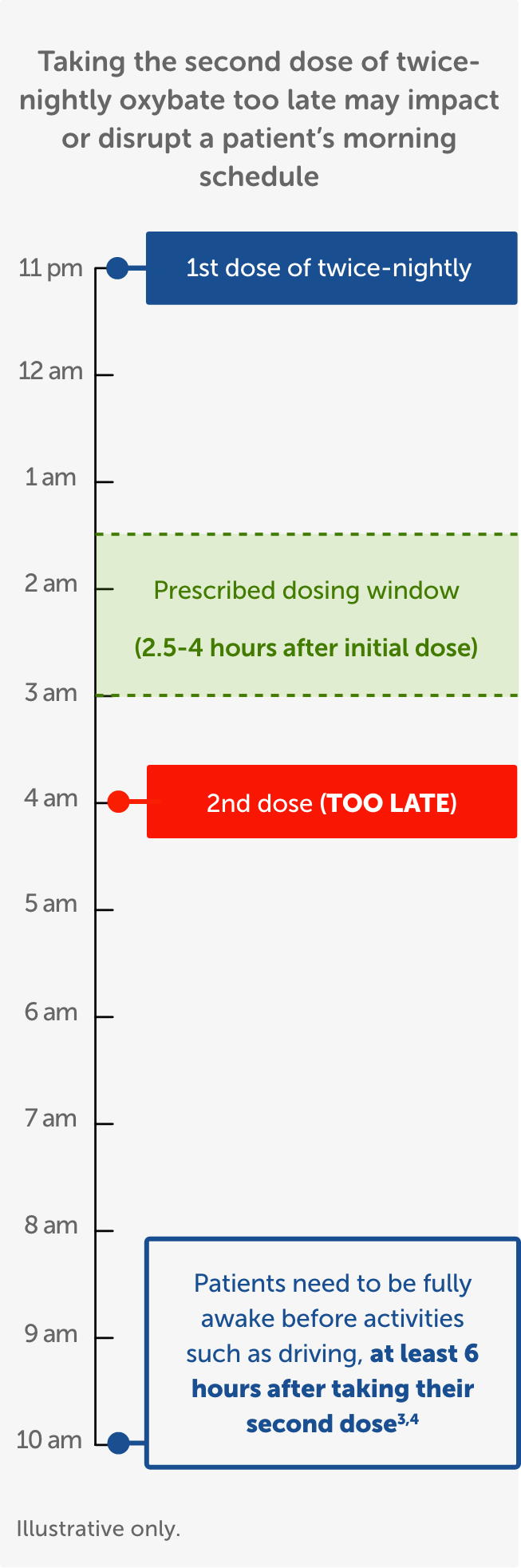
After first initiating treatment and until certain that LUMRYZ does not affect them adversely (eg, impair judgment, thinking, or motor skills), caution patients against hazardous activities requiring complete mental alertness or motor coordination such as operating hazardous machinery, including automobiles or airplanes. Also caution patients against engaging in these hazardous activities for at least six (6) hours after taking LUMRYZ. Patients should be queried about CNS depression-related events upon initiation of LUMRYZ therapy and periodically thereafter.
Abuse and Misuse
LUMRYZ is a Schedule III controlled substance. The active ingredient of LUMRYZ, sodium oxybate, is the sodium salt of gamma-hydroxybutyrate (GHB), a Schedule I controlled substance. Abuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreases in the level of consciousness, coma, and death. The rapid onset of sedation, coupled with the amnestic features of GHB, particularly when combined with alcohol, has proven to be dangerous for the voluntary and involuntary user (eg, assault victim). Physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely.
LUMRYZ REMS
LUMRYZ is available only through a restricted distribution program called the LUMRYZ REMS because of the risks of central nervous system depression and abuse and misuse.
Notable requirements of the LUMRYZ REMS include the following:
- Healthcare providers who prescribe LUMRYZ are specially certified.
- LUMRYZ will be dispensed only by pharmacies that are specially certified.
- LUMRYZ will be dispensed and shipped only to patients who are enrolled in the LUMRYZ REMS with documentation of safe use conditions.
Further information is available at www.LUMRYZREMS.com or by calling 1-877-453-1029.
Respiratory Depression and Sleep-Disordered Breathing
LUMRYZ may impair respiratory drive, especially in patients with compromised respiratory function. In overdoses of oxybate with illicit use of GHB, life-threatening respiratory depression has been reported. Increased apnea and reduced oxygenation may occur with LUMRYZ administration. A significant increase in the number of central apneas and clinically significant oxygen desaturation may occur in patients with obstructive sleep apnea treated with LUMRYZ. Prescribers should be aware that sleep-related breathing disorders tend to be more prevalent in obese patients, in men, in postmenopausal women not on hormone replacement therapy, and among patients with narcolepsy.
Depression and Suicidality
Depression, and suicidal ideation and behavior, can occur in patients treated with LUMRYZ. In an adult clinical trial in patients with narcolepsy (n=212), there were no suicide attempts, but one patient with a history of depression and anxiety developed suicidal ideation in the LUMRYZ-treated patients. The emergence of depression in patients treated with LUMRYZ requires careful and immediate evaluation. Patients with a previous history of a depressive illness and/or a suicide attempt should be monitored carefully for the emergence of depressive symptoms while taking LUMRYZ.
Other Behavioral or Psychiatric Adverse Reactions
Other behavioral and psychiatric adverse reactions can occur in patients taking LUMRYZ. During adult clinical trials in patients with narcolepsy administered LUMRYZ, 2% of 107 patients treated with LUMRYZ experienced a confusional state. No patients treated with LUMRYZ discontinued treatment because of confusion. Anxiety occurred in 7.5% of 107 patients treated with LUMRYZ in the adult trial in patients with narcolepsy. Other psychiatric reactions reported in adult clinical trials in patients with narcolepsy administered LUMRYZ included irritability, emotional disorder, panic attack, agitation, delirium, and obsessive thoughts. Other neuropsychiatric reactions reported in adult clinical trials in patients with narcolepsy administered immediate-release sodium oxybate and in the postmarketing setting for immediate-release sodium oxybate include hallucinations, paranoia, psychosis, aggression, and agitation. The emergence or increase in the occurrence of behavioral or psychiatric events in patients taking LUMRYZ should be carefully monitored.
Parasomnias
Parasomnias can occur in patients taking LUMRYZ. Sleepwalking, defined as confused behavior occurring at night and at times associated with wandering, was reported in 3% of 107 patients with narcolepsy treated with LUMRYZ. No patients treated with LUMRYZ discontinued due to sleepwalking. Episodes of sleepwalking should be fully evaluated and appropriate interventions considered.
Use in Patients Sensitive to High Sodium Intake
LUMRYZ has a high sodium content. In patients sensitive to sodium intake (eg, those with heart failure, hypertension, or renal impairment), consider the amount of daily sodium intake in each dose of LUMRYZ.
MOST COMMON ADVERSE REACTIONS
Most common adverse reactions (incidence ≥5% and greater than placebo) reported for any dose of LUMRYZ were nausea, dizziness, enuresis, headache, and vomiting.
ADDITIONAL ADVERSE REACTIONS
Additional adverse reactions that occurred in ≥2% of patients treated with LUMRYZ and were more frequent in the LUMRYZ treatment group than with placebo were vomiting, nausea, decreased weight, decreased appetite, dizziness, somnolence, headache, enuresis, anxiety, and somnambulism.
Drug Interactions
LUMRYZ is contraindicated for use in combination with alcohol or sedative hypnotics. Use of other CNS depressants may potentiate the CNS-depressant effects of LUMRYZ.
PREGNANCY AND LACTATION
There are no adequate data on the developmental risk associated with the use of sodium oxybate in pregnant women. LUMRYZ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. GHB is excreted in human milk after oral administration of sodium oxybate. There is insufficient information on the risk to a breastfed infant, and there is insufficient information on milk production in nursing mothers. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LUMRYZ and any potential adverse effects on the breastfed infant from LUMRYZ or from the underlying maternal condition.
PEDIATRIC USE
Safety and effectiveness of LUMRYZ in pediatric patients have not been established.
GERIATRIC USE
Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
HEPATIC IMPAIRMENT
LUMRYZ should not be initiated in patients with hepatic impairment because appropriate dosage adjustments for initiation cannot be made with the available dosage strengths. Patients with hepatic impairment who have been titrated to a maintenance dosage of another oxybate product can be switched to LUMRYZ if the appropriate dosage strength is available.
DEPENDENCE AND TOLERANCE
There have been case reports of withdrawal, ranging from mild to severe, following discontinuation of illicit use of GHB at frequent repeated doses (18 g to 250 g per day) in excess of the recommended dosage range. Signs and symptoms of GHB withdrawal following abrupt discontinuation included insomnia, restlessness, anxiety, psychosis, lethargy, nausea, tremor, sweating, muscle cramps, tachycardia, headache, dizziness, rebound fatigue and sleepiness, confusion, and, particularly in the case of severe withdrawal, visual hallucinations, agitation, and delirium. These symptoms generally abated in three (3) to fourteen (14) days. In cases of severe withdrawal, hospitalization may be required. The discontinuation effects of LUMRYZ have not been systematically evaluated in controlled clinical trials. Tolerance to LUMRYZ has not been systematically studied in controlled clinical trials. There have been some case reports of symptoms of tolerance developing after illicit use at dosages far in excess of the recommended LUMRYZ dosage regimen.
Please see full Prescribing Information, including BOXED Warning.
 REQUEST A REPRESENTATIVE
REQUEST A REPRESENTATIVE